Michael DeWine, governor of the state of Ohio in the United States, provided a visible example of the properties of screening tests.
Gov. Mike DeWine of Ohio Tests Positive, Then Negative, for Coronavirus by Sarah Mervosh, The New York Times (6 August 2020 with 7 August 2020 update).
After testing positive for COVID-19 on a rapid antigen test, he missed an opportunity to meet with the US president who was visiting DeWine’s state. After DeWine was tested again using a slower, more accurate (RT-PCR) test, he was negative for COVID-19. A additional test administered a day later also was negative.
Are there benefits in having a rapid, less accurate test as well as having a slower, more accurate test? Let’s consider what accuracy means in these tests and why you might be willing to tolerate different errors at different times.
I won’t address how these tests are evaluating different biological endpoints. I’ve been impressed at how national and local sources have worked to explain the differences between tests that look for particular protein segments or for genetic material characteristic of the virus. Richard Harris (National Public Radio in the US) also provided a nice discussion of reliability of COVID-19 tests that might be of interest.
I want to talk about mistakes, errors in testing. No test is perfectly accurate. Accuracy is good but accuracy can be defined in different ways, particularly in ways that reflect errors in decisions that are made. Two simple errors are commonly used when describing screening tests – saying someone has a disease when, in truth, they don’t (sorry Governor DeWine) or saying someone is disease free when, in truth, they have the disease. Governor DeWine had 3 COVID-19 tests – the first rapid test was positive, the second and third tests were negative. Thus, we assume his true health status is disease free.
These errors are called false positive and false negative errors. (For those of you who took introductory statistics class in a past life, these errors may have been labeled differently: false positive error = Type I error and false negative error = Type II error.) Testing concepts include the complements of these errors – sensitivity is the probability a test is positive for people with the disease (1 – false negative error rate) and specificity is the probability a test is negative for disease-free people (1 – false positive rate). If error rates are low, sensitivity and specificity are high.
It is important to recognize these errors can only be made when testing distinct groups of people. A false positive error only can be made when testing disease-free people. A false negative error only can be made when testing people with the disease. An additional challenge is that the real questions people want to ask are “Do I have the disease if I test positive?” and “Am I disease free if my test is negative?” Notice these questions involve the consideration of two other groups –people who test positive and people who test negative!
Understanding the probabilities
Probability calculations can be used to understand the probability of having a disease given a positive test result — if you know the false positive error rate, the false negative error rate and the percentage of the population with the disease, along with testing status of a hypothetical population. The British Medical Journal (BMJ) provides a nice web calculator for exploring the probability that a randomly selected person from a population has the disease for different test characteristics. In addition, the app interprets the probabilities in terms of counts of individuals from a hypothetical population of 100 people classified into 4 groups based upon true disease status (disease, no disease) and screening test result (positive, negative).
It is worth noting that these probabilities are rarely (if ever) known and can be very hard to estimate – particularly when changing. In real life, there are serious challenges in estimating the numbers that we get fed into calculators such as this – but that’s beyond scope of this post. Regardless, it is fun and educational to play around with the calculator to understand how things work.
These error rates vary between different test types and even for tests of the same type. One challenge that I had in writing this blog post was obtaining error rates for these different tests. Richard Harris (NPR) reported that PCR false positives from the PCR test were approximately 2%, with variation attributable to the laboratory conducting the study and the test. National Public Radio reported that one rapid COVID-19 test had a false negative error rate of approximately 15% while better tests have false negative tests less than 3%. One complicating factor is that error rates appear to depend on when the test is given in the course of disease.
Examples
The following examples illustrate a comparison of tests with different accuracies in communities with different disease prevalence.
Community with low rate of infection
A recent story about testing in my local paper reported 1.4% to 1.8% of donors to the American Red Cross had COVID-19. Considering a hypothetical population with 100 people, only 2 people in the population would have the disease and 98 would be disease free.
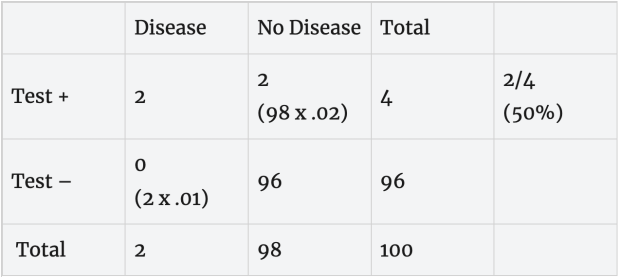
Rapid, less accurate test: Suppose we have a rapid test with a 10% false positive error rate (90% specificity), 15% false negative error rate (85% sensitivity) and 2% of people tested are truly positive. With these error rates, suppose both of the people with the disease test positive and 10 of the 98 disease-free people test positive. Based on this, a person with a positive test (2 + 10= 12) has about a 16% (2/12 x 100) chance of having the disease, absent any other information about exposure.
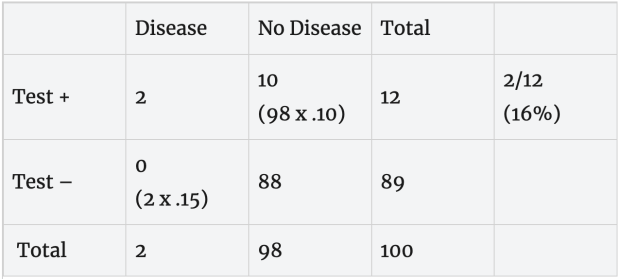
Slower, more accurate test: Now, suppose we have a more accurate test with a 2% false positive error rate (98% specificity) and 1% false negative error rate (99% sensitivity). With these error rates, both of the people with the disease test positive and 2 of the 98 disease-free people test positive. Based on this, a person with a positive test (2 + 2= 4) has about a 50% (2/4) chance of having the disease.
Community with a higher rate of infection
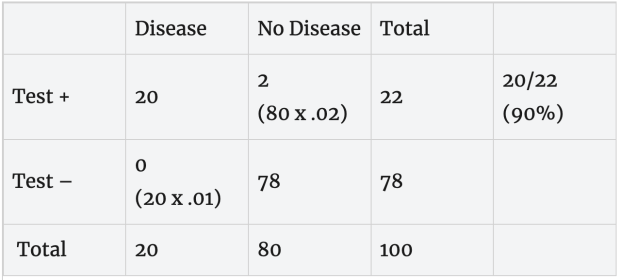
Now suppose we test in a community where 20% have the disease. Here, 20 people in the hypothetical population of 100 have the disease and 80 are disease free. This 20% was based on a different news source suggesting that 20% was one of the highest proportions of COVID-19 in a community in the US.
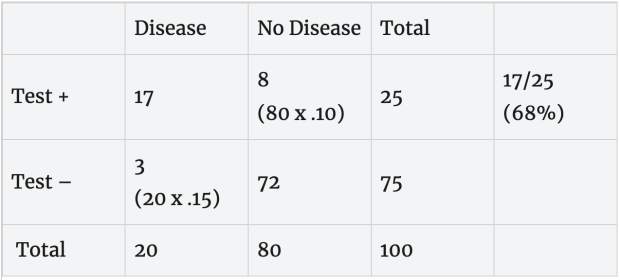
Rapid, less accurate test: Consider what happens we use a rapid test with a 10% false positive error rate (90% specificity) and 15% false negative error rate (85% sensitivity) in this population. With the error rates described for this test, 17 of the 20 people with disease test positive and 8 of the 80 disease-free people test positive. Based on this, a person with a positive test (17 + 8 = 25) has about a 68% (17/25) chance of having the disease without any additional information about exposure.
Slower, more accurate test: Now suppose we apply a more accurate test with a 2% false positive error rate (98% specificity) and 1% false negative error rate (99% sensitivity) to the same population. In this case, all 20 people with the disease test positive and 2 of the 80 disease-free people test positive. Based on this, a person with a positive test (20 + 2 = 22) has about a 90% (20/22) chance of having the disease.
Returning to the big question
Returning to question posed in the title of this blog post …
So, if you test positive for COVID-19, do you have it? If you live in a community with little disease and use a less accurate rapid test, then you may only have a 1 in 6 chance (16%) of having the disease (absent any additional information about exposure). If you have a more accurate test, then the same test result may be associated with a 50-50 chance of having the disease. Here, you might want to have a more accurate follow up test if you test positive on the rapid, less accurate test. If you live in a community with more people who have the disease, both tests suggest you are more likely than not to have the disease. Recognize that these tests are being applied in situations with additional information being available including whether people exhibit COVID-19 symptoms and/or live or work in communities with others who have tested positive.
Final thoughts
You might be interested in controlling different kinds of errors with different tests. If you are screening for COVID-19, you might want to minimize false negative errors and accept potentially higher false positive error rates. A false positive error means a healthy disease-free person is quarantined and unnecessarily removed from exposing others. A false negative error means a person with disease is free to mix in the population and infect others. So, does Governor DeWine have COVID-19? Ultimately, the probability that the governor is disease-free reflects the chance of being disease-free given one positive result on a less accurate test and two negative results from more accurate tests. The probability he is disease-free is very close to one, given no other information about exposure.
To learn more
To read more about natural frequencies in discussing screening test risks:
- Gerg Gigerenzer (2014) Risk Savvy: How to Make Good Decisions. Viking Press.
- Do doctors understand test results? By William Kremer BBC World Service
To read more about natural frequencies / hypothetical populations as part of 7 concepts important for being a statistically literate citizen:
- Jessica Utts (2003) What Educated Citizens Should Know About Statistics and Probability, The American Statistician, 57:2, 74-79, DOI: 10.1198/0003130031630
- The BMJ site include a good exposition of test accuracy and what physicians need to know about these tests. Interpreting a covid-19 test result
To read more about accuracy of COVID-19 tests:
- COVID-19 Story Tip: Beware of False Negatives in Diagnostic Testing of COVID-19 (Johns Hopkins press release describing work suggesting false negative rates > 20% for RT-PCR tests and that test accuracy changes over time course of disease).
- Study Raises Questions About False Negatives From Quick Covid-19 Test from NPR Morning Edition on April 21, 2020.
To read more about screening tests in a different context – facial recognition systems:
- Live Facial Recognition: how good is it really? We need clarity about the statistics by David Spiegelhalter and Kevin Mcconway